. It may additionally be Employed in other programs, which would not have particulate issue specifications, in which bulk H2o for Injection or Purified H2o is indicated but where by usage of a validated drinking water process is not really practical or where somewhat more substantial quantities than are offered as Sterile Drinking water for Injection are desired. Sterile Water for Inhalation— Sterile Water for Inhalation (see USP monograph) is H2o for Injection that may be packaged and rendered sterile and is meant for use in inhalators and from the preparing of inhalation options.
) for lengthier intervals (e.g., 5 to 7 times) can Get well larger microbial counts when put next to classical methods. Small-nutrient media are designed for these decreased temperature and for a longer time incubation circumstances (from time to time so long as 14 days To optimize recovery of really slow increasing oligotrophs or sanitant wounded microorganisms), but even substantial-nutrient media can occasionally boost their Restoration with these more time and cooler incubation circumstances. Whether or not a selected procedure must be monitored applying high- or minimal-nutrient media with increased or reduce incubation temperatures or shorter or lengthier incubation situations must be identified for the duration of or prior to program validation and periodically reassessed as being the microbial flora of a fresh drinking water procedure step by step create a gentle point out relative to its routine servicing and sanitization procedures.
Provider of preformulation, formulation, analytical and personalized pharmaceutical excipients solutions Browse more Grow your awareness, go through our journal directly
takes advantage of materials which might be highly productive deionizers and that don't lead copper ions or organics on the drinking water, assuring an exceptionally high-quality water. In the event the h2o of this purity contacts the ambiance even briefly as it is getting used or drawn from its purification method, its conductivity will promptly degrade, by around about one.0 µS/cm, as atmospheric carbon dioxide dissolves inside the water and equilibrates to bicarbonate ions. As a result, If your analytical use involves that drinking water purity continues to be as large as you can, its use really should be shielded from atmospheric exposure. This water is utilised as being a reagent, being a solvent for reagent preparing, and for test apparatus cleansing where by significantly less pure waters wouldn't conduct acceptably. Nevertheless, if a person's routinely available purified water is filtered and fulfills or exceeds the conductivity specifications of Higher Purity Water, it may be Employed in lieu of Superior Purity Drinking water. Ammonia-No cost Water— Functionally, this drinking water need to have a negligible ammonia focus to avoid interference in tests sensitive to ammonia. It has been equated with Large Purity Drinking water that has a substantially tighter Stage one conductivity specification than Purified H2o due to latter's allowance for a minimum level of ammonium amongst other ions. Even so, If your consumer's Purified Drinking water have been filtered and satisfied or exceeded the conductivity specifications of Large Purity Drinking water, it might incorporate negligible ammonia or other ions and will be Utilized in lieu of High Purity Water. Carbon Dioxide-Totally free check here Water— The introductory portion of the Reagents, Indicators, and Options segment defines this h2o as Purified Water which has been vigorously boiled for a minimum of five minutes, then cooled and shielded from absorption of atmospheric carbon dioxide. Since the absorption of carbon dioxide has a tendency to push down the drinking water pH, most of the employs of Carbon Dioxide-Cost-free Water are either linked being a solvent in pH-connected or pH- sensitive determinations or like a solvent in carbonate-sensitive reagents or determinations. A different use of the drinking water is for particular optical rotation and coloration and clarity of Remedy tests. Although it can be done that this water is indicated for these tests simply because of its purity, it is also feasible which the pH effects of carbon dioxide made up of h2o could interfere with the outcome of these tests. A 3rd plausible motive that this h2o is indicated is the fact outgassing air bubbles might interfere with these photometric-form tests. The boiled h2o planning approach can even tremendously reduced the concentrations of all kinds of other dissolved gases in conjunction with carbon dioxide. As a result, in a lot of the purposes for Carbon Dioxide-Free Water, it could be the inadvertent deaeration outcome that truly renders this h2o appropriate.
Appraise the test outcomes that were entered in possibly logbooks or on loose analytical sheets. Although some brands could possibly be hesitant to deliver tabulations, summaries, or printouts of microbiological test success, this knowledge ought to be reviewed for that identification of likely microbial complications in processing.
The dechlorination procedure could incompletely take out the chloramine, which could irreparably hurt downstream unit functions, but also the discharge of ammonia through this process could carry by way of pretreatment and prevent the concluded h2o from passing compendial conductivity specifications. The purification process should be reassessed When the ingesting drinking water disinfectant is altered, emphasizing the need for a very good Doing work connection amongst the pharmaceutical water company along with the drinking h2o company.
Microbial contamination in biological medicines can cause significant wellbeing challenges for clients, together with bacterial infections, septicemia, and other adverse reactions. Consequently, it's essential to carry out microbial limit test to make sure the protection and high-quality of your drug products.
This concern is talked over in detail down below. The 2nd thought will be the incubation problems. Exceptional situations for growth have to be existing to make sure full development and reproducible effects.
Retest final results ought to be reviewed and website evaluated, and certain emphasis ought to be placed on the logic and rationale for conducting the retest.
Execute the resolve less than conditions meant to avoid extrinsic microbial contamination of the item to become examined.
Equivalent recovery among the test group along with the peptone team demonstrates ample neutralizer efficacy; very similar Restoration amongst the peptone group along with the viability group demostrates ample neutralizer toxicity.
Inspect the autoclaves useful for the sterilization of media. Autoclaves may perhaps deficiency a chance to displace steam with sterile filtered air. For sealed bottles of media, This is able to not existing a challenge. On the other hand, for non-sealed bottles or flasks of media, non-sterile air has led into the contamination of media. On top of that, autoclaving less than the demanded time can even let media linked contaminants to increase and result in a Bogus constructive end result. These troubles could be a lot more common in laboratories using a hefty workload.
Due to the fact warn and motion degrees need to be according to real procedure functionality, along with the process general performance data are created by a given test method, it follows that those warn and motion degrees need to be legitimate just for test benefits produced by precisely the same test method. It really is invalid to apply alert and motion stage standards to test final results generated by a different test method.
Amongst An important components of the inspection of a sterility analytical software should be to evaluation documents of initial optimistic sterility test effects. Ask for lists of test failures to aid evaluation of creation and Regulate documents and investigation reviews. Notably, with the high danger aseptically stuffed merchandise, First favourable sterility test results and investigations needs to be reviewed.
 Haley Joel Osment Then & Now!
Haley Joel Osment Then & Now! Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now! Destiny’s Child Then & Now!
Destiny’s Child Then & Now!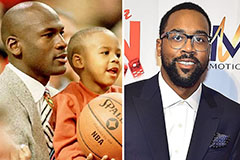 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Suri Cruise Then & Now!
Suri Cruise Then & Now!